
Great Chemistry.
Manufacturing processes of immunoglobulin preparations may differ, resulting in differences in the final product composition. Immunoglobulin safety is GC Biopharma’s primary goal for our formulation.
A longstanding goal within the immunoglobulin (IG) industry has been to remove coagulation factor XIa (FXIa), a plasma-based protein linked to IVIG-related thromboembolic events.
An extra step in our process dedicated to the removal of FXIa
- Our manufacturing process includes multiple steps, such as Cohn-Oncley, cold-ethanol fractionation, viral inactivation, and nanofiltration
- We implemented an extra step in our manufacturing process using CEX chromatography specifically to address the need to remove FXIa
- An in depth study was designed to evaluate GC Biopharma's manufacturing process and it's capacity to remove FXIa
- The study demonstrated that CEX chromatography effectively removed >99% of FXIa
The CEX chromatography difference
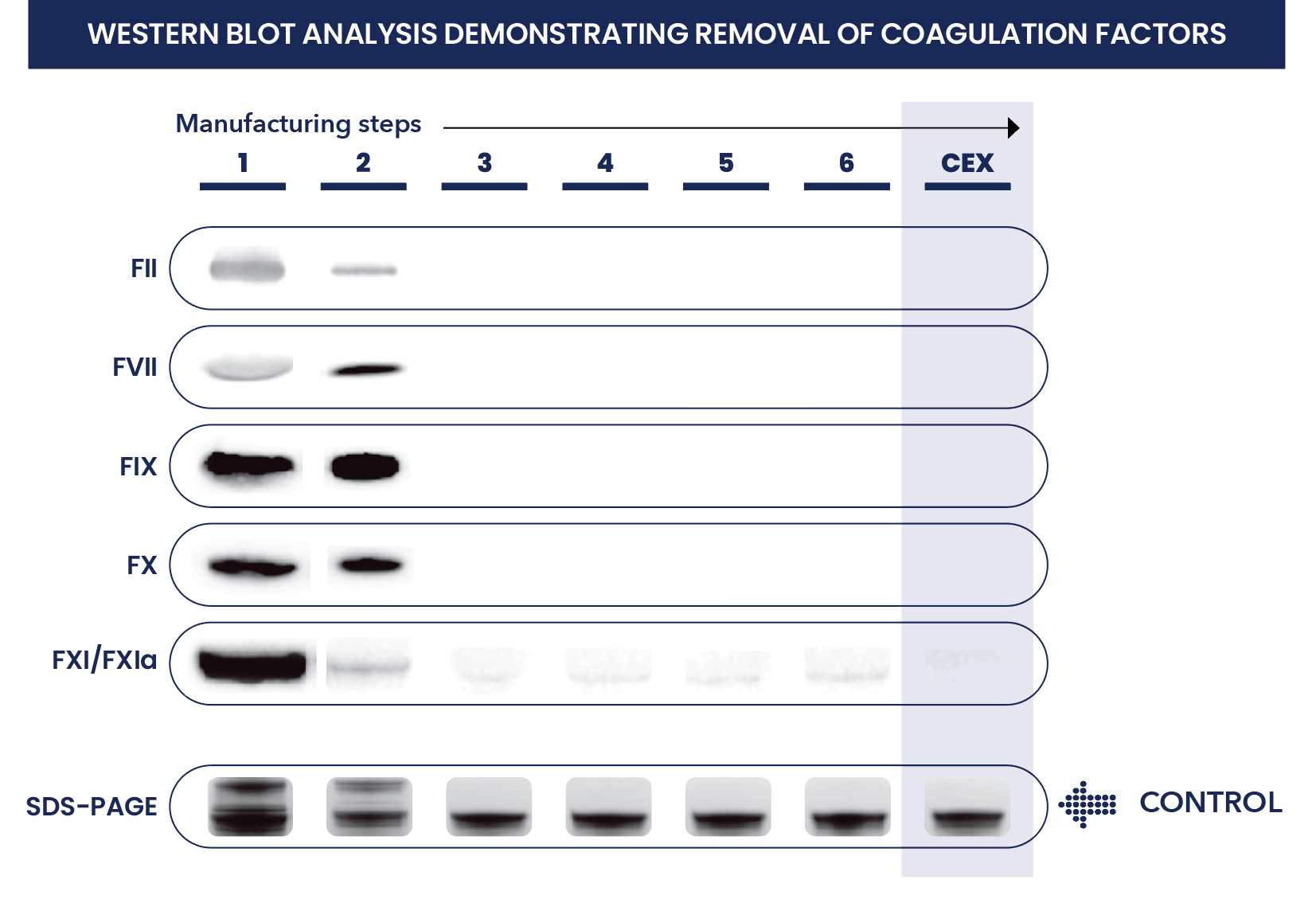
- Coagulation factors (FII, FVII, FIX, FX, and FXIa) remaining in IVIG products at high concentrations can potentially cause thromboembolic events. FXIa is known to play an important role in causing thrombosis with IVIG
- Ethanol precipitation can effectively remove coagulant factors FII, FVII, FIX, and FX but FXIa can remain in intermediary products
- Western blot and ELISA analyses demonstrated that residual FXIa remained in the intermediate manufacturing product until after CEX chromatography, when it was reduced to undetectable limits
How CEX Chromatography works
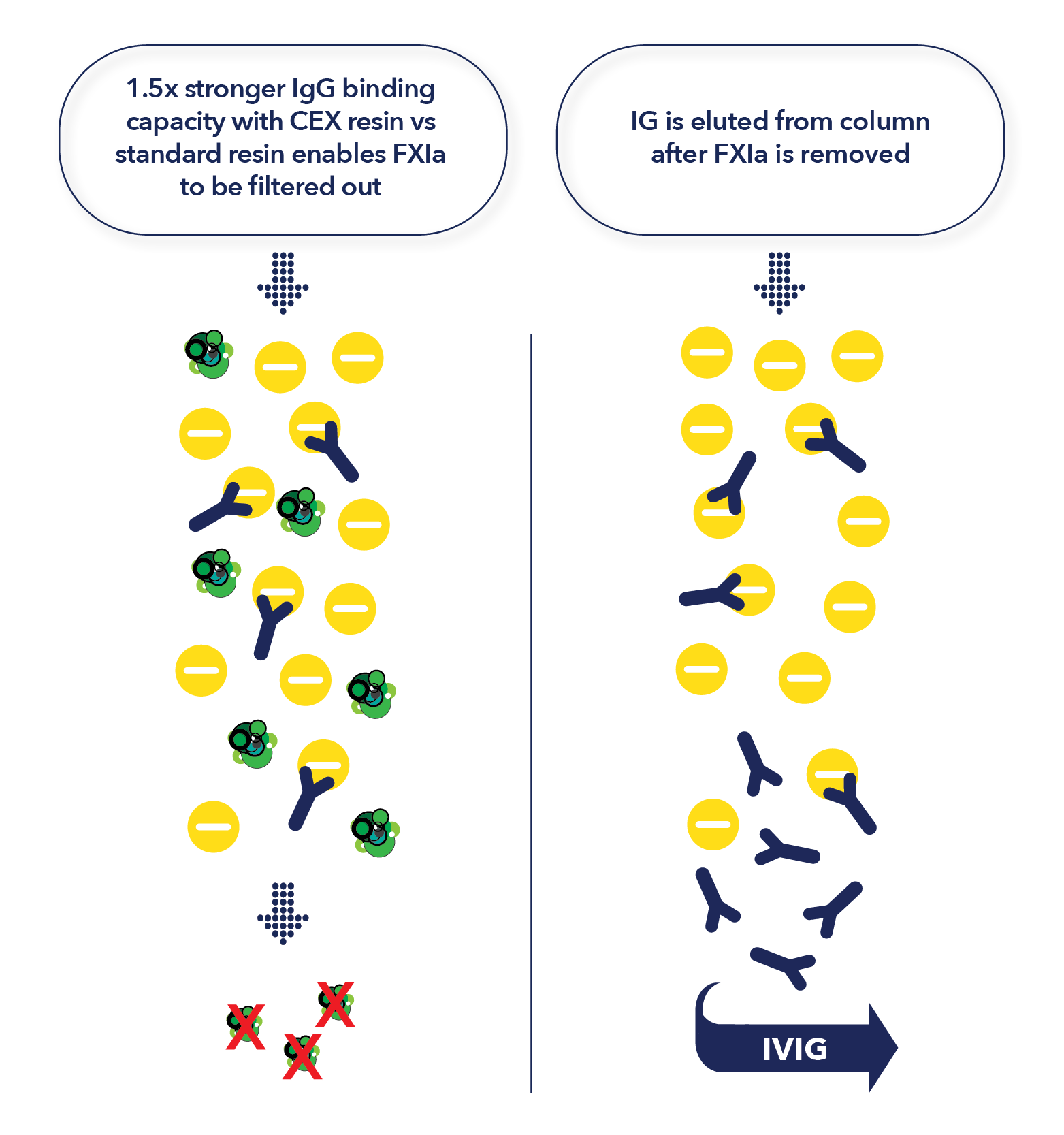
- Human IgG has an isoelectric point ranging from 6.4 to 9.0, while the isoelectric point for FXIa ranges from 8.9 to 9.1
- Because of this similarity, it can be difficult to separate FXIa from IgG preparations using ethanol precipitation alone
- CEX chromatography, using a unique ceramic resin under specific conditions, allows the FXI and Ig molecules to be separated.
- While FXIa binds tightly to the resin, purified IgG flows through the column
- FXIa is then removed from the column in the “Clean in Place” step only after the purified IgG has been collected
Tested and proven to reduce FXIa to undetectable levels.
- A spiking study was designed to evaluate GC Biopharma’s IVIG manufacturing process for its capacity to remove coagulation FXIa
- In the study, IVIG samples were spiked with large quantities of FXIa (32-fold and 158-fold) before the CEX chromatography step in manufacturing
- FXIa levels were measured after CEX chromatography using an enzyme-linked immunosorbent assay
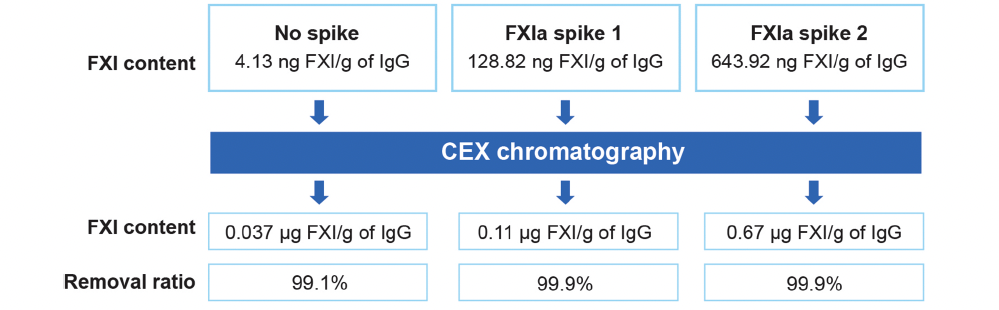
The spiking study demonstrated that CEX chromatography removed >99% of FXIa protein and reduced FXI activity to below detection limits, even in samples containing FXIa levels 158x greater than what you would normally see.
Reference: Kang GB, Huber A, Lee J, et al. Cation exchange chromatography removes FXIa from a 10% intravenous immunoglobulin preparation. Front Cardiovasc Med. 2023;10:1253177.